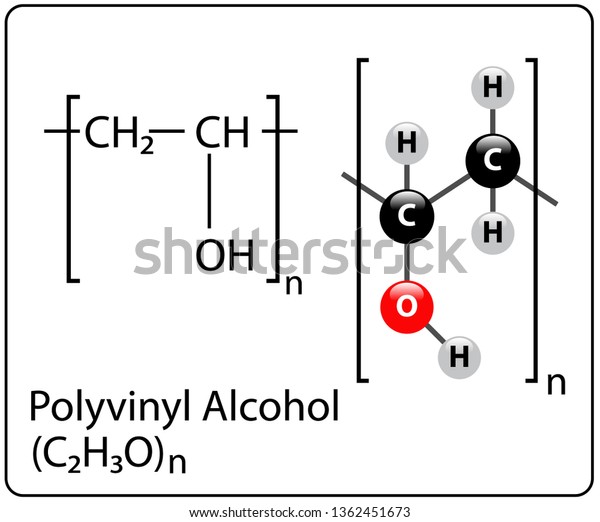
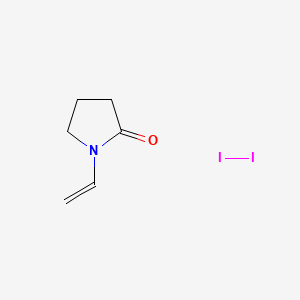
Skeletal Structure Of Vinyl Alcohol
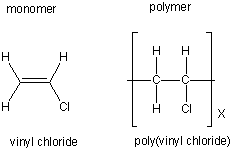
An industrially important example is vinyl chloride precursor to pvc a plastic.
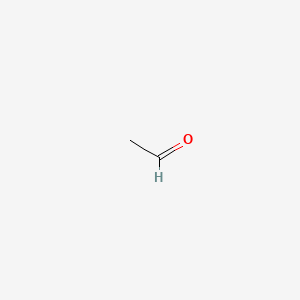
Skeletal structure of vinyl alcohol. Alcohols are consumed as beverages where the alcohols specifically consist of 3 40 per cent of ethanol by volume. This geometrical arrangement reflects the effect of electron repulsion and the increasing steric bulk of the substituents on the central oxygen atom. When the reaction is allowed to proceed to completion the product is highly soluble in water and insoluble in practically all organic solvents. Vinyl alcohol can be formed by the pyrolytic elimination.
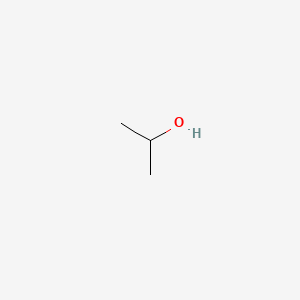
See chemical bonding for a discussion of hybrid orbitals alkyl groups are generally bulkier than hydrogen atoms however so the r o h bond angle in. It is not a precursor to polyvinyl alcohol synthesis. Vinyl alcohol also called ethenol iupac name is the simplest enol. Similar to water an alcohol can be pictured as having an sp3 hybridized tetrahedral oxygen atom with nonbonding pairs of electrons occupying two of the four sp3 hybrid orbitals.
A skeletal structure does not show carbon or hydrogen atoms explicitly nor does it show the c h bonds. Alcohol alcohol structure and classification of alcohols. Some are listed below. So oct tells us that we have 8 carbons.
Component of watersoluble edible film that may be used to form pouches containing pre portioned aliquots of 1 certain dry ingredients i e instant tea instant coffee hot chocolate mix flavored drink powder and whey protein supplement powder to be used by the consumer in preparing ready to serve foods and beverages at a. The chemical structure of the resulting vinyl alcohol repeating units is. One at each end and one at the intersection of the two line segments. Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
Incomplete removal of the acetate groups yields resins less soluble in water and more soluble in certain organic liquids. Now we have to specify where that triple bond is. These are used as an anti freezing agent with a mix of a solution containing ethylene glycol dissolved in water. Structure and physical properties of alcohols.
The structure of an alcohol is similar to that of water as it has a bent shape. But because we have an alcohol there we want to call this an octyne let me make it very clear. With the formula c h 2 choh it is a labile compound that converts to acetaldehyde. In chemistry vinyl or ethenyl abbreviated as vi is the functional group with the formula c h ch 2 it is the ethylene iupac ethene molecule h 2 c ch 2 with one fewer hydrogen atom.
Alcohol ethanol is used as an antiseptic agent. The name is also used for any compound containing that group namely r ch ch 2 where r is any other group of atoms. There are several uses of alcohols. You always specify the lower number of the carbons on that triple bond.
The triple bond is on the 5 carbon. So it is oct 5 yn. Like water alcohols are polar containing an unsymmetrical.